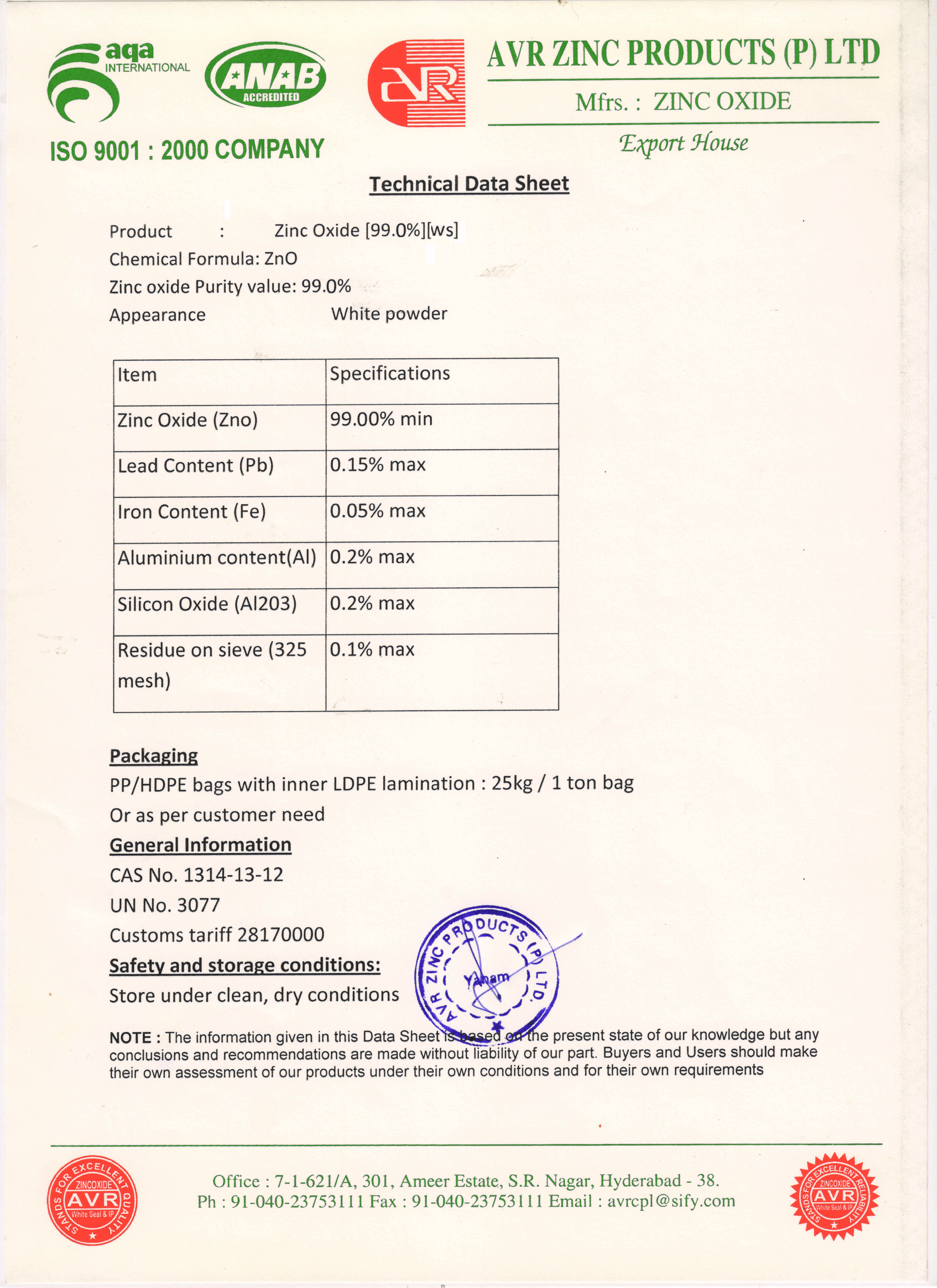
Zinc Oxide
Zinc oxide is an inorganic compound with the formula ZnO. ZnO is a white powder that is insoluble in water. Although it occurs naturally as the mineral zincite, most zinc oxide is produced synthetically.
Properties
- It is a semiconductor
- Good transparency
- high electron mobility
- wide band gap
- Strong room-temperature luminescence.
- High refractive index
- high thermal conductivity
- UV-protection properties
Properties
For industrial use, zinc oxide is produced by three main processes:
Indirect process
In the indirect or French process, metallic zinc is melted in a graphite crucible and vaporized at temperatures above 907 °C (typically around 1000 °C). Zinc vapor reacts with the oxygen in the air to give ZnO, accompanied by a drop in its temperature and bright luminescence. Zinc oxide particles are transported into a cooling duct and collected in a bag house. This indirect method was popularized by LeClaire (France) in 1844 and therefore is commonly known as the French process.
Direct process
The direct or American process starts with diverse contaminated zinc composites, such as zinc ores or smelter by-products. The zinc precursors are reduced by heating with a source of carbon such as anthracite to produce zinc vapor, which is then oxidized as in the indirect process. Because of the lower purity of the source material, the final product is also of lower quality in the direct process as compared to the indirect one.
Wet chemical process
A small amount of industrial production involves wet chemical processes, which start with aqueous solutions of zinc salts, from which zinc carbonate or zinc hydroxide is precipitated. The solid precipitate is then calcined at temperatures around 800 °C.
Applications
- Rubber manufacture
- Ceramic industry
- Medicine
- Cigarette filters
- Food additive
- Pigment
- UV absorber
- Coatings
- Corrosion prevention in nuclear reactors

Tulsiram Hanumanbagas Gilada (THG), is a leading trade house for Synthetic Rubbers and Rubber Chemicals. Established and Lead by Mr Ramakant Gilada 3 decades ago at Hyderabad and later expanded to all over South India.
Contact Us
- thghyd@gmail.com
- +91 7893883141
Copyright © 2024 Tulsiram HanumanBagas Gilada. All Rights Reserved.